Clinical Roundup: Gene-Edited CAR T Therapies
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
In 2017, the American Food and Drug Administration (FDA) approved the very first CAR T-cell therapy, Kymriah, for the treatment of B-cell acute lymphoblastic leukaemia (B-ALL).
As of February 2021, four patient-derived CAR T therapies had been approved by the FDA to treat various blood cancers, and today there are more than 1,000 ongoing clinical trials evaluating CAR T technology for new treatments with better safety, efficacy and the potential to treat solid cancers.
CAR T technology has revolutionised cancer treatment for patients with certain blood cancers that are refractory to other treatments, with remission rates above 90% in some types of leukaemia. However, despite a very promising start for this new class of therapies, a number of therapy-associated limitations remain, including: poor CAR T cell persistence due to T cell fratricide, logistical and economic barriers to production and quality control of patient-derived CAR T cells, long time to infusion of CAR T cells which may reduce overall outcome, and suboptimal T cell quality when isolated from patients who have already been treated with lymphotoxic chemotherapies.
Gene editing can augment CAR T-cell therapies
To address the current limitations and augment the efficacy of CAR T therapies, researchers have turned to gene-editing technologies such as CRISPR-Cas, transcription activator-like effector nucleases (TALENs) and meganucleaases.
Today, these gene-editing modalities are used in various ways to develop novel donor-derived ‘off-the-shelf’ universal CAR T therapies with increased persistence, safety and efficacy, and which may also withstand the risk of relapse due to antigen loss in certain cancers.
This roundup looks at how the varoius gene-editing technologies are used to advance and augment the CAR T therapies that are currently in clinical trials.
The need for precise CAR insertion into T cell genome
For the four approved CAR T therapies, the CARs are inserted into the genome of patient-isolated T cells by means of transduction with either a retroviral or a lentiviral vector that harbours the target-specific CAR.
Although integrating viruses are a popular choice for many gene therapy applications, some concerns exist about their safety. One issue with respect to CAR T therapies is that viral transduction doesn’t allow site-specific insertion of the CAR construct, which could result in insertion within a tumour suppressor gene or oncogene and lead to cancer. Although this has not been reported for CAR T therapy so far, the possibility to control where CARs are inserted into the genome is highly desirable. Using precise gene editing to insert the CAR directly rather than using integrating viruses is expected to result in safer, more consistent CAR T products.
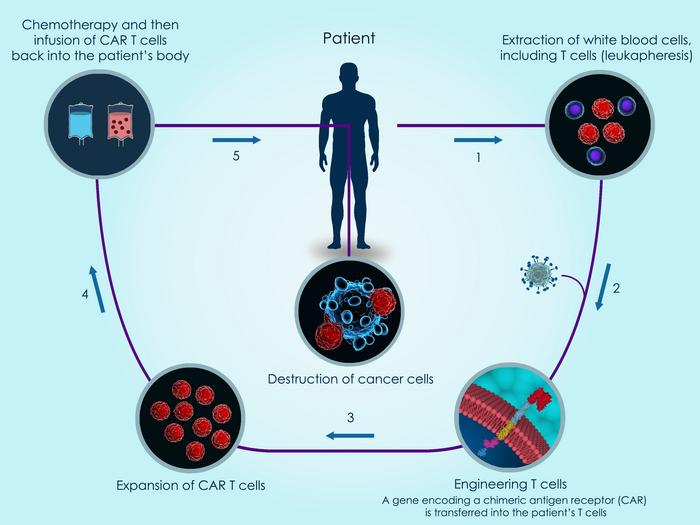
CAR T-cell therapy in brief
The principle behind CAR T therapy is that T cells are engineered with a so-called chimeric antigen receptor (CAR) that allows the cells to selectively target cancer cells bearing the target that is recognised by the CAR.
A CAR is a synthetic transmembrane protein that contains two key domains: one that binds to the target antigen, most often a cell surface receptor that is abundant on diseased cells but not on healthy cells, and another that activates the T cell upon antigen recognition.
To develop a CAR T therapy, cells from either the diseased individual (autologous therapy) or healthy donors (allogeneic therapy) are isolated and engineered to express a CAR. The most widely used CARs target CD70, CD19 or B-cell maturation antigen (BCMA). Gene-edited CAR T therapies are further engineered using strategies described in this article. The engineered cells are then either transfused back into the individual to be treated or cryopreserved in off-the-shelf vials.
While the approved CAR T therapies are all made from patient-isolated cells, many of the CAR T therapies in clinical development are based upon donor-derived T cells. Such allogeneic strategies provide a larger source of cells to work with, making it possible to develop standardised off-the-shelf therapies, and saving patients from undergoing blood cell isolation procedures.
CRISPR-Cas edited CAR T therapy candidates
The pipeline of CRISPR Therapeutics contains three CRISPR-edited CAR T therapies (CTX110, CTX120 and CTX130) in five ongoing Phase 1/2 clinical trials for refractory and relapsed B and T cell cancers, multiple myeloma (MM) and renal cell carcinoma (RCC).
CRISPR Therapeutics employs CRISPR-Cas9 to insert either a CD19-, B-cell maturation antigen- (BCMA) or CD70-targeting CAR into the TCR alpha constant (TRAC) locus in the T cell genome of donor-derived T cells. TRAC harbours the native T cell receptor (TCR) gene and inserting the CAR in its place has a number of advantages.
Researchers have previously reported that replacing the endogenous TCR with an CD19-specific CAR for leukaemia was advantageous since it knocked out existing TCRs while knocking in CAR19 under the regulatory control of the endogenous TCR promoter, leading to improved T cell function and potency in mouse models of ALL. Disruption of the native TCR also mitigates the risk of graft versus host disease (GvHD) that may result from histoincompatibility between a donor and recipient when allogeneic cell therapies are used.
Great Ormond Street Hospital for Children NHS Foundation Trust (UK) is sponsoring a paediatric trial for relapsed or refractory B-ALL in collaboration with University College London. This trial is evaluating the safety and efficacy of an allogeneic CAR T candidate known as TT52CAR19. This candidate is transduced with a lentivirus to express a CD19-targeting CAR. CRISPR-Cas9 technology is used disrupt CD52 and the native TCR receptor gene TRAC in the TT52CAR19 cells. These edits mitigate the risk of graft vs host disease, and render the therapeutic cells resistant to the anti-CD52 monoclonal antibody alemtuzumab, which is used during lymphodepletion, respectively.
Fate Therapeutics is developing FT819 as a CD19-specific CAR T therapy for refractory and relapsed B cell cancers. Besides CRISPR-mediated insertion of the CAR at the TRAC locus which is seen in several other CAR T candidates, FT819 is somewhat unique as the first-ever CAR T-cell therapy to be derived from a clonal master iPSC line engineered with Fate Therapeutic's proprietary iPSC platform. Caribou Bioscience's CD19 CAR T candidate CB-010 for relapsed and refractory B cell non-Hodgkin lymphoma is also worth mentioning since it contains an edit not seen very widely in CAR T clinical-stage candidates. CB-010 is edited with CRISPR to delete the PD-1 gene. This gene encodes the PD-1 protein which functions as a safety switch on T cells that cancer cells turn on to protect themselves from T cell-mediated immune responses. You can read more about Fate Therapeutics and Caribou Bioscience's gene-edited CAR T programmes in our recent post.
TALENs are also used to augment CAR T Therapies
Institut De Recherches Internationales Servier, an international research company and part of the Servier Group, headquartered in France, is sponsoring two Phase 1 trials using allogeneic gene-edited CAR T candidates for B-cell acute lymphoblastic leukaemia (B-ALL) in adults and children. Here, TALENs are deployed to develop the CD19-targeting candidate, UCART19, in which the native TCR is also deleted. As an inbuilt safety feature, the CD20 portion of the CAR permits selective depletion of the UCART19 cells when the anti-CD20 monoclonal antibody rituximab is administered, in the case of unacceptable adverse effects of UCART19. UCART19 also contains another transgene, RQR8, which is engineered to contain epitopes from CD34 and CD20, which allows tracking of the UCART19 cells with a clinically approved anti-CD34 antibody.
Allogene Therapeutics is sponsoring three Phase 1 clinical trials for its TALEN-engineered CAR T therapies ALLO-316, ALLO-501 and ALLO-715 for the treatment of RCC, non-Hodgkin lymphoma (NHL) and MM, respectively. Integrating, self-inactivating recombinant lentivirus is used to express the therapeutic CARs in these candidates. These ALLO- candidates are developed using Allogene's AlloCAR T platform, which deploys TALEN technology (under licence from Cellectis) to knock out both the TRAC locus and the CD52 gene. Knocking out TRAC eliminates native TCR expression at the cell surface which reduces the risk of GvHD, while knocking out CD52 enables expansion of the edited cells and renders them resistant to lympodepletion with the anti-CD52 monoclonal antibody ALLO-647, which is used to deplete host T cells.
Cellectis is sponsoring three universal allogeneic CAR T candidates from its own pipeline for B-ALL, acute myeloid leukaemia (AML) and MM. Recruitment is currently ongoing to evaluate these candidates for safety in Phase 1 trials. In all cases, TALEN technology is used to disrupt the native TCR, and disease-specific CARs targeting either CD22 (B-ALL), CD123 (AML) or CS1 (MM) are expressed via lentiviral vectors.
Meganuclease-editing of CAR T candidates
Precision Biosciences is sponsoring four Phase 1 or 2 clinical trials for four meganuclease-edited CAR T therapies for the treatment of MM, ALL, NHL, Chronic Lymphocytic Leukaemia (CLL) and Small Lymphocytic Lymphoma (SLL). These candidates PBCAR0191, PBCAR269A, PBCAR20A and PBCAR19B are developed using Precision BioSciences’ next-generation meganuclease platform ARCUS. This platform produces meganucleases with customised activity and specificity, which are capable of distinguishing target sites that differ by only 1 base pair.
PBCAR19B is a so-called 'stealth cell' anti-CD19 CAR T product that is designed to prevent immune rejection by recipients. In addition to targeted CAR insertion and simultaneous disruption of the TRAC locus it bears two additional genetic modifications that are expected to boost its persistence. The vector used to edit PBCAR19B cells carries a short hairpin RNA that suppresses beta-2 microglobulin (B2M) expression, which in turn eliminates the expression of all MHC I molecules on CAR T cells, reducing the risk of self-presenting to recipient T cells, and allowing persistence in their new host. CRISPR Therapeutics previously reported positive pre-clinical effects of CRISPR-mediated B2M suppression for its BMCA-targeting CAR T candidate for MM.
PBCAR19B cells are also engineered to express the conserved HLA-E transgene. While B2M disruption eliminates all MHC I molecules to boost persistence, it also leaves the CAR T cells vulnerable to natural killer (NK) cells. HLA-E surface expression in the absence of all other MHC molecules prevents NK-mediated lysis without stimulating allogeneic T cells.
Precision Biosciences' other three clinical-stage CAR T candidates are engineered to express a CD19-, BCMA- or CD20-targeting CAR in place of the native TCR gene.
A fast moving area
The CAR T and gene-editing fields are advancing fast and new trials as well as trial updates are emerging continuously. In this roundup we have tried to summarise the main gene edits seen in clinical-stage CAR T therapies. Many of the trials are at an early stage and the exact gene-editing strategies have not been disclosed in many cases. We have also not covered every CAR T trial in this update. We will continue to bring updates as they emerge. In the meantime, you can check out our CRISPR Medicine News Clinical Trials Database for a complete overview of gene-editing clinical trials.
This roundup was originally published on 31st March 2021. In the original text, it was incorrectly stated that Allogene Therapeutics and Cellectis are using TALEN technology to insert a CAR into the TRAC locus. These inaccuracies were corrected on 6th February 2023. This amendment affects the second and third paragraphs of the section entitled: 'TALENs are also used to augment CAR T Therapies'.
Tags
ArticleNewsin vivoAcute Lymphoblastic Leukemia, ALLAcute Myeloid Leukemia, AMLB-cell Malignancy, NHLMultiple Myeloma, MMMultiple Solid Tumor AdultNon-Hodgkin Lymphoma, NHLRenal Cell CarcinomaT-Cell LymphomaCAR-TADIR-SERVIERUniversity College, LondonCRISPR-CasCas9MeganucleasesTALENsAllogene Therapeutics, Inc.Caribou Biosciences, Inc.Cellectis S.A.CRISPR Therapeutics AGFate Therapeutics, Inc.Great Ormond Street Hospital for Children NHS TrustInstitut De Recherches Internationales Servier Iris, SARL.Trials
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







