Trends That Advanced CRISPR Therapies in 2021
CMN Intelligence - The World’s Most Comprehensive Intelligence Platform for CRISPR-Genomic Medicine and Gene-Editing Clinical Development
Providing market intelligence, data infrastructure, analytics, and reporting services for the global gene-editing sector. Read more...
In case it has skipped anybody's attention, CRISPR is moving fast in the medical field, and 2021 saw lots of innovation and progress. In this roundup, we have selected three technological trends that CRISPR Medicine News has covered extensively in the past year, and we briefly summarise the progress that has been made.
Gene editing without double-strand breaks
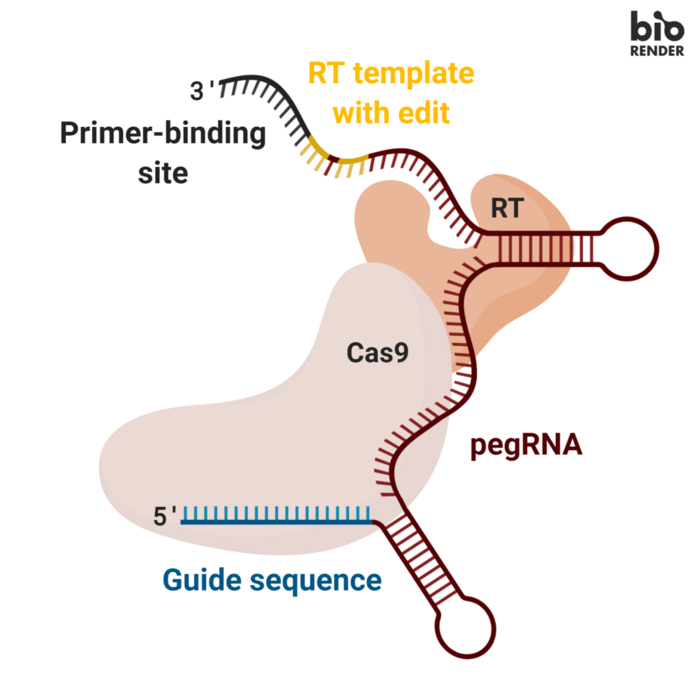
A hallmark of traditional CRISPR-Cas9 gene editing is the creation of a DNA double-strand break at the nuclease target site. This is, however, also one of the major concerns with the technique - especially in the clinical setting - since it can lead to undesired repair outcomes, genome instability and activation of oncogenes.
Fortunately, David Liu at Broad Institute developed two alternative techniques to overcome these challenges: base editing in 2016 and prime editing in 2019. Both approaches rely on fusion enzymes consisting of a modified Cas9 that binds to the target site but only nicks one of the two DNA strands and an enzyme that chemically modifies the genetic code.
November 2021 marked the first time a base-editing therapy moved towards the clinic when Beam Therapeutics got FDA approval for an Investigational New Drug (IND) application to treat sickle cell disease (SCD). The BEAM-101 therapy utilises base editing to reactivate the expression of foetal haemoglobin in autologous haematopoietic stem cells and is envisioned as a one-time treatment for SCD. In addition, proof of concept for another attempt to treat SCD in mice with base editing by correcting the faulty β-globin gene was presented by David Liu in June.
While both these achievements use transplantation of ex vivo base-edited cells, 2021 was also the year when in vivo base editing became mainstream in preclinical studies with mice. This approach was used to treat blindness (Leber congenital amaurosis, LCA) by Susie Suh at University of California, Irvine, the premature ageing disease progeria by David Liu, and Duchenne muscular dystrophy by Eric Olson at the University of Texas Southwestern.
In another first for 2021, the lab of Hyongbum Henry Kim at the Yonsei University in Seoul demonstrated successful in vivo prime editing in mice. Using this approach, the group corrected both mutations and phenotypes in mice suffering from a genetic liver disease, hereditary tyrosinemia, and blindness caused by LCA.
Targeting pathogenic bacteria with phages and conjugation
In recent years, it has become clear that the microbiome is crucial for human health and that an imbalance in the human microbial biodiversity can lead to several diseases. These include antibiotic-resistant infections and inflammatory bowel disease, obesity, type 2 diabetes, hypertension, colorectal cancer, and even Alzheimer's disease.
This fact has sparked the idea that CRISPR could be used therapeutically without editing human cells but instead targeting specific pathogenic bacteria. While phage therapy has long been used to eliminate pathogenic bacteria with some degree of specificity, loading the phages with CRISPR reagents can add another layer of specificity by targeting a gene only present in the undesired bacterial species or strain.
However, the world's first clinical trial of CRISPR enhanced phage therapy does not target the gut but rather E. coli infections in the lower urinary tract. In February, Locus Biosciences announced promising preliminary data from the trial of LBP-EC01 that utilises Cas3. When the target is encountered, the nuclease moves along one strand of the DNA and shreds it, effectively eliminating the pathogenic bacteria.
The way for another clinical trial was paved just past New Year when SNIPR Biome got FDA clearance for its first IND application. The experimental oral medication, SNIPR001, is designed to treat potentially life-threatening E. coli infections in patients with cancer in the blood, bone marrow or lymph nodes. It contains four E. coli-targeting phages armed with CRISPR reagents that selectively eradicate target bacteria in a sequence-specific manner. Preclinical studies have demonstrated its efficacy in animal disease models.
More CRISPR therapies aiming at pathogenic bacteria are on their way. In November, at the University of California, San Francisco, Peter Turnbaugh used M13 bacteriophages to deliver CRISPR-Cas9 reagents to E. coli within the mouse gastrointestinal tract. The proof of concept approach targeted a fluorescent marker in engineered E. coli that had been seeded in the gut.
In October, an alternative delivery method was demonstrated by Sébastien Rodrigue's group at Université de Sherbrooke in Canada. They used bacterial conjugation to transfer Cas9 and gRNAs targeting an antibiotic-resistance gene from a probiotic donor E. coli strain to resistant strains of E. coli and Citrobacter rodentium in the mouse intestinal tract.
Less is more: Cas nucleases are getting smaller

In the beginning, researchers only thought of Cas9. Then they started to discover a steady stream of other Cas nucleases, each of them having specific features. For example, some target RNA or single-stranded DNA rather than double-stranded DNA, some have a collateral activity that unspecifically degrades DNA, and others come with different requirements for PAM sequences. While researchers can utilise all these differences for specific purposes, there is another varying characteristic, namely size, that increasingly attracts researchers' interests.
Cas9 is one of the biggest known Cas nucleases with 1368 amino acids (aa), while variants of Cas14 go as low as around 400 aa. Size is important because most delivery platforms have a limited cargo capacity. For example, this applies to one of the most popular platforms for in vivo delivery, adeno-associated virus (AAV). AAV uses 90% of its carrying capacity for Cas9 and a gRNA, leaving virtually no room for additional elements like, e.g., a deaminase or reverse transcriptase used for base editing or prime editing, respectively.
Mammoth Biosciences is one of the CRISPR-based companies that has specialised in utilising compact CRISPR systems like Cas14 and CasΦ for its - so far undisclosed - pipeline of in vivo therapies. The company puts a lot of effort into discovering novel small Cas nucleases with unique characteristics, and so do many researchers around the world. In September 2021, Stanley Qi and his group from Stanford University took the quest into their own hands and engineered a multifunctional Cas nuclease, CasMINI, only half the size of Cas9.
Other researchers aim to characterise the minuscule Cas nucleases to reveal how they can still operate despite being so small. This is the case of Guillermo Montoya from the University of Copenhagen in Denmark. In July, his group solved the structure of CasΦ in complex with a DNA target after cleavage, and this might pave the way for future optimisation of this and other Cas nucleases.
May 2022 take CRISPR even further
Apart from all the progress in the three technological trends described here, 2021 has also been a tremendous year for CRISPR on its clinical path to actually improve patients' lives. Let’s hope that 2022 will see lots of new exciting research and clinical results that can take CRISPR even further.
Tags
ArticleNewsin vivoIn vivoAdeno-associated virus (AAV)CancerDuchenne Muscular Dystrophy, DMDE. coli infectionsHereditary BlindnessLeber Congenital AmaurosisBase editorsCas14Cas9CasΦPrime editorsFDABeam Therapeutics Inc.LOCUS Biosciences, Inc.Mammoth Biosciences, Inc.SNIPR BiomeClinicalIND - Investigational New DrugPre-clinical
CLINICAL TRIALS
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.
Sponsors:
Base Therapeutics (Shanghai) Co., Ltd.







