CRISPR Medicine in 2021 – What a Phenomenal Year of Firsts!
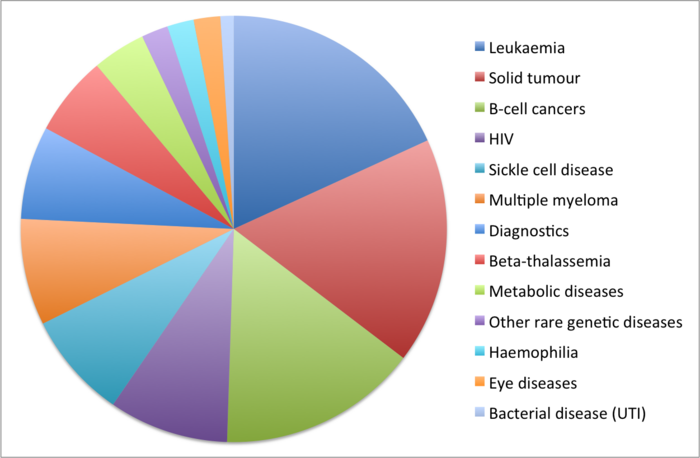
Less than a decade after CRISPR was first used to edit mammalian DNA, the first CRISPR-based cures are getting closer to a reality with 100+ ongoing gene-editing trials for new therapies and diagnostics.
This year, we've seen positive clinical data for the first in vivo CRISPR medicines for rare diseases. New trials are starting for HIV, diabetes and hereditary angiodema. The number of gene-editing cancer trials has increased dramatically, with the proportion of solid tumour candidates also rising thanks to advances in gene-editing technologies. Brand new diseases are entering the CRISPR arena, and ongoing trials are by and large living up to expectations.
It truly is an exciting time for genomic medicine! Join us as we look back on some of the biggest highlights from the CRISPR medicine field this year.
First in vivo CRISPR medicines are efficacious and safe
Landmark clinical data for NTLA-2001 in hereditary transthyrein amyloidosis
This summer, Intellia Therapeutics and Regeneron shared clinical data that support the safety and efficacy of in vivo CRISPR genome editing in humans. This major news related to a Phase 1 trial for NTLA-2001, which has been developed as a single-dose treatment for hereditary transthyretin (TTR) amyloidosis with polyneuropathy (ATTRv-PN). In ATTR, a protein known as TTR becomes misfolded and accumulates as plaques in tissues throughout the body. This causes complications that mainly involve the heart and nerves and which are often fatal. Hereditary and wild-type forms of the disease collectively affect between 200,000 and 500,000 people worldwide, and most patients die 2-15 years after disease onset.
NTLA-2001 was the first ever CRISPR therapy to be injected directly into humans via the bloodstream. It helps patients by selectively lowering the levels of mutated TTR protein in the blood, through CRISPR-Cas9-based inactivation of the TTR gene in liver cells. You can find a summary of NTLA-2001’s mechanism of action in an earlier clinical news article.
So far, Intellia and Regeneron have released data on the first six patients treated from cohorts in New Zealand and the UK. These patients were treated by a single injection with either of two doses of NTLA-2001, and reductions in the range of 52 % - 87 % compared to baseline TTR measurements were observed in all cases. No major safety concerns had presented as of Day 28 post-treatment. During the data announcement, the companies disclosed that enrolment was ongoing for a third cohort who would receive a higher dose. The study is expected to be complete in 2024.
Intellia received U.S. FDA Orphan Drug Designation for NTLA-2001 for ATTR in October 2021, and the company since announced expansion of the Phase 1 trial to include adults with Transthyretin Amyloidosis with Cardiomyopathy (ATTR-CM).
Early but encouraging data for EDIT-101 in Leber congenital amaurosis 10
In September 2021, Editas Medicine shared positive initial clinical data from its ongoing Phase 1/2 BRILLIANCE trial for Leber congenital amaurosis 10 (LCA10), an incurable hereditary eye disease that leads to blindness by the 3rd or 4th decade of life.
EDIT-101 is a candidate CRISR-Cas9-based therapy that is designed to correct LCA10-mutations in the CEP290 gene, which together account for about 20-30 % of all LCA cases. Although CEP290 mutations disable light-detecting cells in the retina, the retinal cells themselves are still present and viable in LCA10, which raises hopes that EDIT-101 can improve vision in this patient group.
What we know about patients treated with EDIT-101 so far is that no serious adverse events or dose-limiting toxicities have been observed, and modest signs of visual improvements have been reported for the mid-dose cohort. At the time of the announcement, the company was continuing to treat a high-dose adult cohort and would begin dosing paediatric patients.
Although it's still early days for EDIT-101, signs of efficacy and safety provide new hopes for LCA, a disease group that represents a severely unmet medical need with poor to modest treatment options that are only available to a subset of patients with the ‘right’ mutations (you can read more about LCA in our earlier disease roundup).
Base editors are headed for the clinic
By far one of the top stories in CRISPR medicine this year was the news that Beam Therapeutics received the FDA’s green light for its base editor candidate BEAM-101 to enter a clinical trial in sickle cell disease (SCD).
BEAM-101 is a patient-specific, autologous blood stem cell therapy designed as a one-shot cure for SCD and the related disease beta-thalassemia (BT), both of which are characterised by a deficiency in functional adult haemoglobin.
The strategy behind BEAM-101 is that patient-derived blood stem cells are base edited ex vivo to incorporate certain point mutations that occur naturally in some healthy individuals who continue to produce foetal haemooglobin beyond infancy. This therapeutic strategy is not new (read more about BEAM-101 and the other six gene-editing therapies for SCD here) but it is the first to involve a base editor.
The hope is that base editors will offer safe correction of genetic diseases that are caused by a single-point mutation, since they make precise single-base changes without introducing double-strand breaks, unlike conventional CRISPR nucleases. Remarkably, the IND approval of BEAM-101 comes just five years after base editors were developed in the lab of Dr. David Liu’s lab at Broad Institute – who is also a co-founder of Beam Therapeutics.
If base editing lives up to current expectations, it will be a major turning point for rare disease sufferers. Of the >50,000 mutations that are linked with human disease, 33,739 are single-point mutations. Findings from experiments in animal diseases models already make a compelling case for the potential of base editors to correct several rare genetic diseases including progeria, Leber congenital amaurosis, SCD, and Duchenne muscular dystrophy.
New diseases enter the CRISPR arena
The range of diseases that are being addressed in gene-editing clinical trials has expanded greatly in 2021. In the last year alone, the number of trials for gene-editing therapies has risen to just under 100, and we are now seeing new therapies emerging for diseases that weren’t previously in scope, such as diabetes and hereditary angioedema, the first CRISPR-based candidate for HIV, as well as an increase in the number of trials for solid tumours.
Diabetes
Although diabetes doesn’t fall into the category of rare diseases and or/poorly treated diseases that often dominate CRISPR developmental pipelines, a CRISPR-based therapy is expected to offer a cure to the many millions of type 1 diabetes (T1D) and type 2 diabetes (T2D) sufferers worldwide, which would eliminate the need for daily insulin injections and reduce the massive healthcare burden associated with current treatment options.
CRISPR Therapeutics and ViaCyte announced in November that their joint T1D candidate VCTX210 had passed the IND approval stage in Canada, and the companies were planning to start enrolment before year end. VCTX210 is a stem cell-derived therapy made from healthy donor cells, and is designed as a best-in-class treatment for T1D and insulin-dependant T2D. The candidate is designed to replace the beta cells that are lost in diabetes, and is developed using a CRISPR-Cas9 gene-editing approach that involves disruption and insertion. Read our original news article about VCTX210 here.
Hereditary angioedema
Hereditary angioedema (HAE) is another 'new' disease that entered the gene-editing arena this year. This painful disease is characterised by severe inflammatory attacks with swelling in various organs and tissues. Plasma kallikrein is a protein that is known to drive multiple inflammatory pathways including the inflammatory processes seen in HAE.
In October, Intellia Therapeutics announced that its CRISPR-based therapy for HAE had passed the approval stage for clinical trial inititon in New Zealand. In the Phase 1/2 trial, NTLA-2002 is adminstered directly into the body and is designed to knock out the target gene kallikrein B1 (KLKB1) in liver cells, which is expected to permanently reduce plasma kallikrein activity and halt the inflammation seen in HAE. This is the first ever gene-editing candidate for HAE to enter a clinical study, and the first patient was dosed earlier this month.
First CRISPR-Cas9 in vivo candidate for HIV
Excision BioTherapeutics is developing EBT-101 as a potential cure for chronic HIV infection, and it announced in September that the FDA had approved its IND application to begin a clinical trial for HIV-1, which is the subtype present in about 95 % of HIV cases worldwide. This is the first CRISPR-based approach to treat HIV to reach the clinic. In the Phase 1 trial, EBT-101 will be delivered directly to the body via an adeno-associated virus (AAV) as a one-time treatment that is designed to cut the HIV virus out of infected cells. Read more about EBT-101 here.
Happy New Year to all!
2021 has been a fantastic year for CRISPR medicine, and as the year draws to a close, we thank you all for your continued support and engagement to date.
What will happen in 2022? Will more base editors progress to the clinic? Will we witness the first approvals for CRISPR-, TALEN-, zinc finger nuclease or meganuclease-edited therapies? And will newer gene-editing technologies such as prime editing advance to clinical stage?
We are certain that 2022 has many exciting advances in store and we are looking forward to continue to bring you the news!
In this article, we've only covered a fraction of the news updates from this year in CRISPR medicine. You can find all our previous news articles about IND approvals and clinical trials here. For a complete overview of CRISPR IND approvals and ongoing gene-editing trials, check out CRISPR Medicine News' Clinical Trials Database.
To get more of the CRISPR Medicine News delivered to your inbox, sign up to the free weekly CMN Newsletter here.
Tags
ArticleNewsin vivoIn vivoRibonucleoprotein (RNP)Adeno-associated virus (AAV)DiseaseBlood DiseaseCancerDiabetesDuchenne Muscular Dystrophy, DMDHaemophiliaHereditary angioedema, HAEHereditary BlindnessHuman Immunodeficiency Virus Infection, HIVHutchinson-Gilford Progeria SyndromeInfectious diseaseLeber Congenital AmaurosisMetabolic disorderMultiple Myeloma, MMMultiple Solid Tumor AdultSickle Cell Disease, SCDSolid Tumor AdultSolid TumoursT-Cell LymphomaTransfusion-Dependent Beta Thalassemia, TDTTransthyretin amyloidosis, ATTRTransthyretin Cardiac Amyloidosis, TTRType 1 diabetesType 2 diabetesUrinary Tract Infections, UTIDisease Trial CardsBacterial InfectionBlood diseaseCancerMetabolic disorderRare DiseaseReagentsBase editorsCas-CLOVERCRISPR-CasCas9MeganucleasesPrime editorsTALENsZFN - Zinc finger nucleasesFDAHealth CanadaBeam Therapeutics Inc.Editas Medicine, Inc.Excision BioTherapeuticsIntellia Therapeutics, Inc.ViacyteTrialsClinical
CLINICAL TRIALS
Sponsors:
Suzhou Maximum Bio-tech Co., Ltd.
Sponsors:
Zhejiang University







